Which Term Best Describes All Atoms in Ionic Bonds
Ionic bond is formed by the complete transfer of electrons between atoms. Metals gain electrons and nonmetals lose electrons to form bonds.
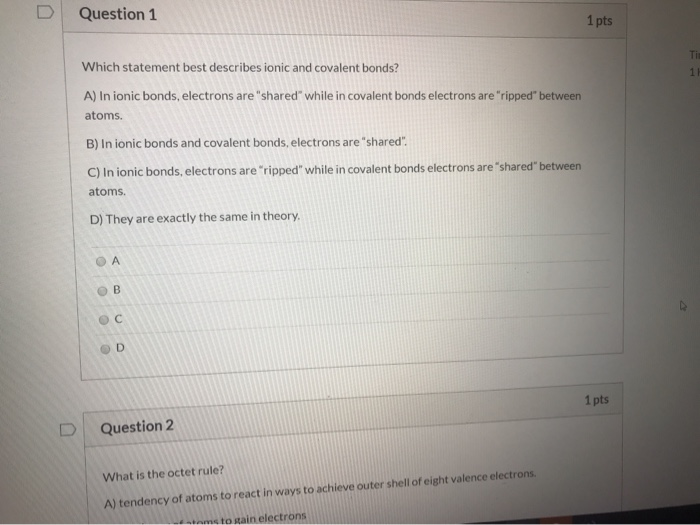
Solved Question 1 1 Pts Which Statement Best Describes Ionic Chegg Com
The cation are positively charged and an anions are negatively charged.

. Valence electrons are transferred from one atom to the other. O atoms breaking bonds O ions breaking bonds water molecules. Dmitriy789 7 6 months ago.
The covalent bond is a bond formed when two atoms share one or more electron pairs. You might be interested in. Both metals and nonmetals gain electrons to form bonds.
1 Dissociation refers to a general process in which molecules break up in to atoms ions or free radicals. It is made up of 11 total atoms of 3 different elements. Which term best describes all atoms in ionic bonds.
Which term best describes all atoms in ionic bonds. Which statement best describes how an existing theory is often affected by the development of new technology. The atoms valence electrons are shared between the atoms.
4 Which answer below best describes the type of bond occurs between two oxygen atoms. Using these tools Lionel can test several properties of an ionic compound. Mila 183 1 year ago.
Which term best describes all atoms in ionic bonds-stable-unstable-positively charged-negatively charged. View the full answer. Which of the following best describes the way ionic bonds are formed.
Particle O Atom O Molecule O Substance. Ionic bonds are stable. Last two options options c and d basically refer to solva.
What does the chemical formula C3H6O2 tell about a molecule of the compound it represents. Which of the following terms best describes dissociation. 3 What term describes the bond between atoms in a molecule of oxygen.
Ionic compounds are the compounds which are made up of an ions that are cations and an anions. Each atom contributes an equal number of electrons towards the bond formation. These adaptations are developed over time in order to help the organism to be best suited to its environment to obtain food and water as well as to avoid predators.
Stable unstable positively charged negatively charged. Lionel has a hot plate a thermometer a container of water a spoon and a small hammer. Ionic compound.
The correct option is A Stable. Which term best describes all atoms in ionic bonds. Question 27 3 points Which term best describes an equal sharing of a bonded pair of electrons between atoms non-polar covalent bond dipole moment octet rule polar covalent bond ionic bond Get the answer to your homework problem.
Which term best describes all atoms in ionic bonds. Which term best describes all atoms in ionic bonds. Ionic compounds are the compounds which are made up of an ions that are cations and an anions.
5 How would you describe the bond that exists between two oxygen atoms to make the O2 molecule. Which of the following terms best describes dissociation. Which word best describes this.
What should be the ending of the second element in a covalent compound. How many valence electrons are in an atom of fluorine. However he will be unable to test the compounds.
Which statement correctly describes a covalent bond. The ionic bond is the attraction between positive and negative ions in a crystal and compounds held together by ionic bonds are called ionic compounds. Oxygen o How do the nuclei of covalently bonded atoms help keep the bond together.
What is produced during the replacement reaction of CuNO32 and Zn. Which term best describes all atoms in ionic bonds. Atoms breaking bonds O ions breaking bonds water molecules breaking bonds water molecules surrounding ions Which of the following best describes the reason ionic substances dissolve O ions break bonds O positive ions are attracted to negative ions water molecules are attracted.
Any metals in the reference table can form an ionic bond with a. 1 How the bond between two oxygen atoms is formed. The atoms valence electrons combine to form a network of bonds.
All of the atoms electrons are shared between the atoms. Metals lose electrons and nonmetals gain electrons to form bonds. The element sulfur S is most likely to form covalent bonds with the element.
Both metals and nonmetals lose electrons to form bonds. The ionic bonds are stable when cation and an anions both approaches to each other by electrostatic force they form ionic bonding. What atoms form an ionic bond-.
2 What is the bond between 2 oxygen atoms. The cation are positively charged and an anions are negatively charged.
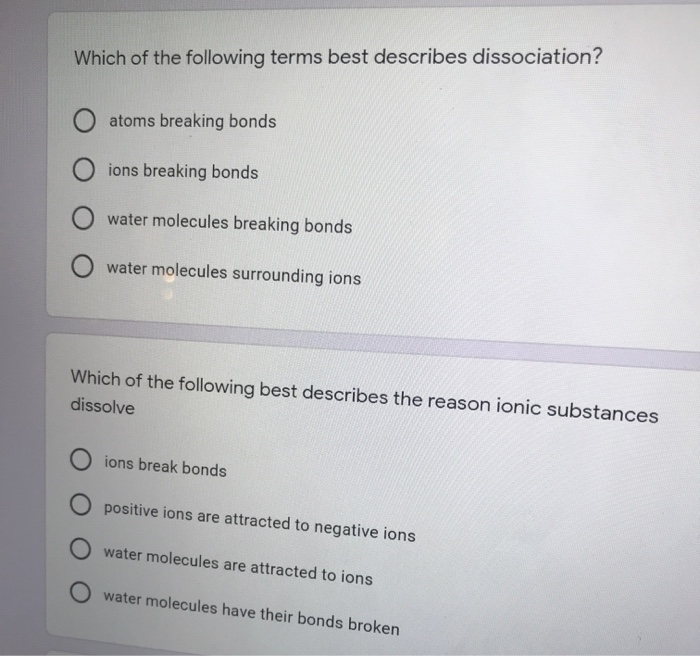
Solved Which Of The Following Terms Best Describes Chegg Com

Question Video Selecting The Statement That Does Not Describe Ionic Bonding Nagwa

Question 6 Of 10 Look At The Picture Below Which Best Describes The Bond Shown A A Covalent Bond Brainly Com
0 Response to "Which Term Best Describes All Atoms in Ionic Bonds"
Post a Comment